Abstract
Background
In pediatric patients with ALL, completion of ASNase treatment has been shown to improve survival (Silverman LB et al. Blood . 2001;97(5):1211-1218). Premedication has been used to reduce infusion and hypersensitivity reactions associated with ASNase therapy; however, premedication may mask the appearance of systemic hypersensitivity reactions, which may be associated with the development of neutralizing antibodies and reduced ASNase activity (Woods D et al. J Pediatr Oncol Nurs . 2017. doi:10.1177/1043454217713455). In the US, 2 formulations of asparaginase are available, a PEGylated form (PEG) of Escherichia coli- derived ASNaseand an immunologically distinct Erwinia chrysanthemi- derived form (ERW). Reported here are results of a physicians' survey conducted via market research to assess the effects of premedication on completion of ASNase treatment.
Methods
An online market research survey of US hematology/oncology physicians collected information on their treatment of pediatric ALL patients since the beginning of 2014. A patient chart audit was also included. Recruited physicians were required to have 2-35 years in practice, ≥75% of time in direct patient care with 80% compliance with treatment using Children's Oncology Group, Dana-Farber, St. Jude, or other pediatric ALL protocols. Patients (aged 1-21 years) were required to be personally managed by a physician, currently receiving or had received treatment for ALL. Physician practice setting affiliation (academic vs community), ASNase use, premedication (premed vs no-premed) use, ASNase-therapy interruption due to complications (physician-assessed hypersensitivity reaction [HSR] or other toxicity) and subsequent treatment decisions (PEG rechallenge, switch to ERW), and rates of therapy completion were also collected. Data collected were descriptive in nature and no inferential statistical tests were performed.
Results
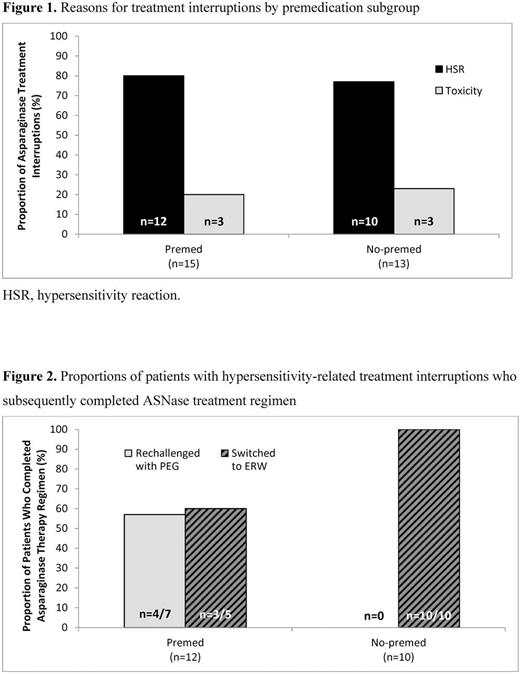
Eighty-five hematologists/oncologists (44% academic, 56% community) provided audited charts for 323 patients (median age, 14 years; 62% classified as high or very-high risk). For the 231 patients receiving PEG as initial ASNase therapy, premedication for the first and/or subsequent doses was used in 151 patients (65%). Premedications included antihistamines (60%), acetaminophen (59%), antiemetics (48%), and steroids (39%). Among the premed patients (n=151), 15 patients had ASNase treatment interrupted, 12 (80%) due to physician-assessed HSR. Among the no-premed patients (n=80), 13 patients had treatment interrupted, 10 (77%) due to physician-assessed HSR (Figure 1). These rates were higher than those for discontinuation due to toxicities other than HSR (3/15, 20% vs 3/13, 23%). For the premed patients with HSR, 5 were switched to ERW, and 7 were rechallenged with PEG. For the no-premed group, all 10 patients were switched to ERW. In patients with HSR, overall completion rates at time of audit were lower in the premed group (58%; 7/12) than in the no-premed group (100%; 10/10; 1 patient in each group was still on therapy at time of audit; Figure 2).
Conclusions
In this chart audit, the majority of pediatric ALL patients treated with PEG as initial ASNase therapy received premedication (65%). Hypersensitivity-like reaction was the most common physician-reported reason for ASNase treatment interruption, even in premedicated patients (80% of treatment interruptions). Patients without premedication had a higher rate of ASNase treatment completion than those who were premedicated. These results should be interpreted with caution given the retrospective nature of this chart audit, the subjective nature of HSR determination, and the small patient numbers.
Support: Jazz Pharmaceuticals.
Bernard: Jazz Pharmaceuticals: Employment. Rustay: Jazz Pharmaceuticals: Employment, Other: stock options. Mamedov: Jazz Pharmaceuticals: Employment, Other: stock options.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal